Quick links to the Trials
FRESH UP Trial
This ACC 2025 study questions the common recommendation of fluid restriction for chronic heart failure. Liberal fluid intake showed no significant harm and even improved patient comfort.
DAPA TAVI Trial
This breakthrough trial shows dapagliflozin significantly reduces heart failure worsening in elderly post-TAVI patients, supporting its use in high-risk groups.
FAIR HF2 Trial
FAIR-HF2 is testing IV iron’s impact on heart failure outcomes. Could treating iron deficiency cut hospital stays and mortality in HFrEF patients?
1. FRESH UP TRIAL
Liberal fluid intake versus fluid restriction in chronic heart failure.
Fluid restriction is frequently recommended to patients with chronic heart failure, but randomized clinical trials assessing the effects of fluid restriction remain scarce.
In this multicenter open-label trial, outpatients with chronic heart failure were randomized to receiving advice for liberal fluid intake versus receiving advice for fluid restriction, up to 1,500 ml per day of fluid intake.
The primary outcome of the trial was health status after 3 months, as assessed by the Kansas City Cardiomyopathy Questionnaire Overall Summary Score (KCCQ-OSS).
Secondary outcomes included thirst distress and safety events. Among 504 randomized patients (67.3% male), the KCCQ-OSS after 3 months was 74.0 in the liberal fluid intake group versus 72.2 in the fluid restriction group, with a mean difference after adjustment for baseline scores of 2.17 (95% confidence interval -0.06 to 4.39; P = 0.06), indicating that the primary outcome was not met.
Thirst distress was higher in the fluid restriction group and no differences were observed for safety events between the two groups.
These findings question the benefit of fluid restriction in chronic heart failure.

2. DAPA TAVI TRIAL
Rationale and trial of Dapagliflozin after Transcatheter Aortic Valve Implantation (DAPA TAVI) Randomized Trial.
The DapaTAVI trial demonstrated that daily dapagliflozin (Farxiga) significantly reduced the incidence of mortality or worsening heart failure (HF) at one year following transcatheter aortic valve implantation (TAVI).
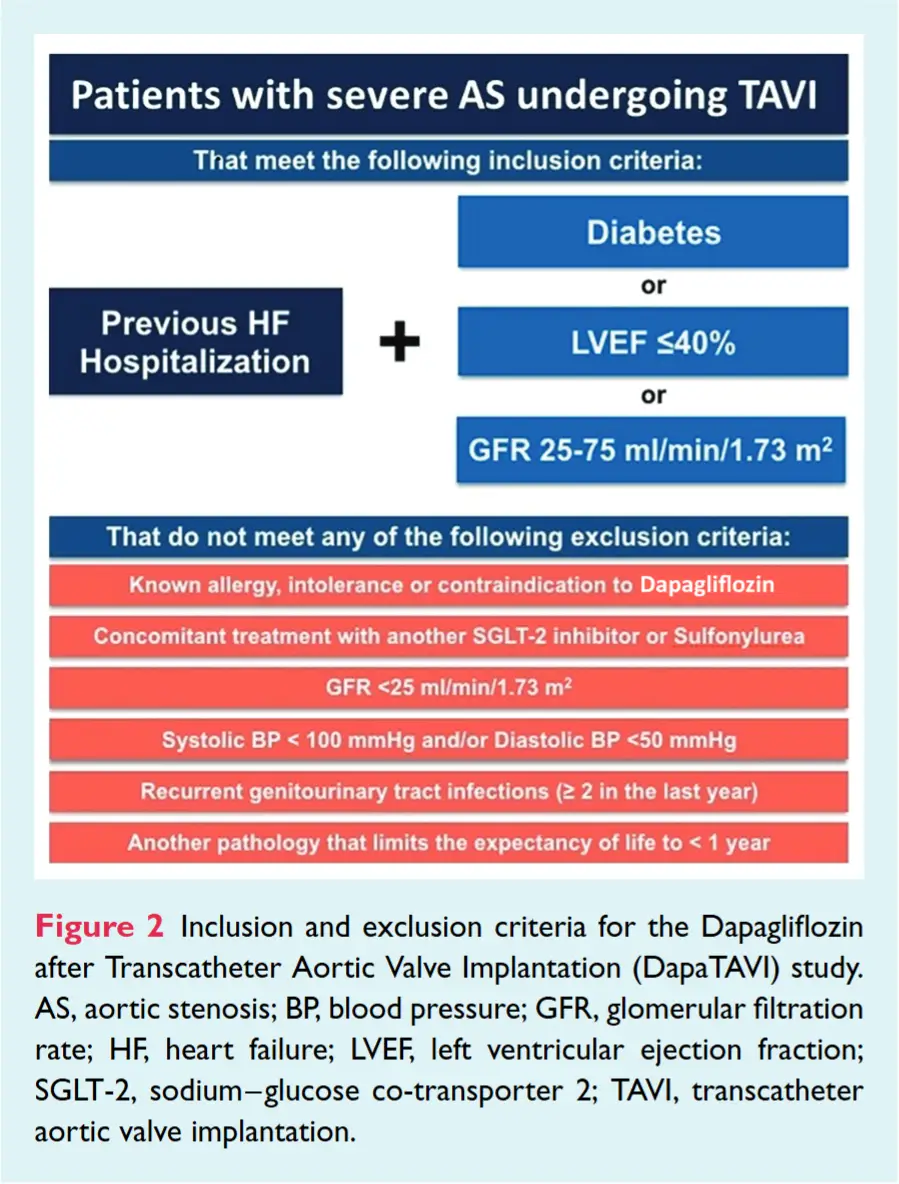
In this randomized trial conducted across 39 centers in Spain, 1222 patients with HF and severe aortic stenosis (AS) were assigned to either dapagliflozin 10 mg or standard care.
The primary outcome of the trial was health status after 3 months, as assessed by the Kansas City Cardiomyopathy Questionnaire Overall Summary Score (KCCQ-OSS).
The primary endpoint—a composite of all-cause mortality or worsening HF—was 28% lower in the dapagliflozin group (15.0%) versus standard care (20.1%) (HR, 0.72; 95% CI, 0.55 to 0.95; P = .02), largely driven by a 37% reduction in worsening HF events.
While overall mortality differences were not statistically significant, dapagliflozin was well tolerated in this elderly population (mean age 82), though it was associated with higher rates of genital infections and hypotension.
These results support the use of SGLT2 inhibitors in high-risk HF patients undergoing TAVI, expanding evidence for their benefit in previously underrepresented populations.

3. FAIR HF 2 TRIAL
Rationale & Design of the FAIR-HF2-DZHK05 Trial: Ferric Carboxymaltose assessment of morbidity & mortality in patients with iron deficiency & chronic heart failure.
FAIR-HF2 is an investigator-initiated, multicentre, randomized, double-blind, placebo-controlled trial that has recruited 1105 patients with chronic HF with a left ventricular ejection fraction of ≤45% and concomitant ID, defined as serum ferritin <100 ng/ml or serum ferritin 100–299 ng/ml with a transferrin saturation <20%.
Patients were consented and randomized to receive either IV FCM (treatment) or saline (placebo).
During an estimated median follow-up of over 2 years, patients underwent a placebo-controlled repletion and maintenance phase, with an initial iron supplementation of up to 2000 mg, followed by 500 mg every 4 months unless stop criteria of haemoglobin >16 mg/dl or serum ferritin >800 ng/ml are met on repeat visits.
The trial will evaluate three primary hypotheses: (i) time to first event of cardiovascular death or hospitalization for HF, (ii) the rate of total (first and recurrent) HF hospitalizations (both analysed in the full study population), and (iii) the time to first event of cardiovascular death or hospitalization for HF in patients with a transferrin saturation <20% at baseline.
The familywise type I error rate across the three primary endpoint hypotheses will be controlled using the Hochberg procedure (alpha 0.05).
The FAIR-HF2 will evaluate the efficacy of FCM in patients with HFrEF in improving cardiovascular outcomes by utilizing a more aggressive approach towards iron supplementation ensuring prevention of transitional ID after initial repletion targets have been met.


